Retatrutide Update: UK Mounjaro Price Shock, Lilly’s AI Deal & PN-477 Rival
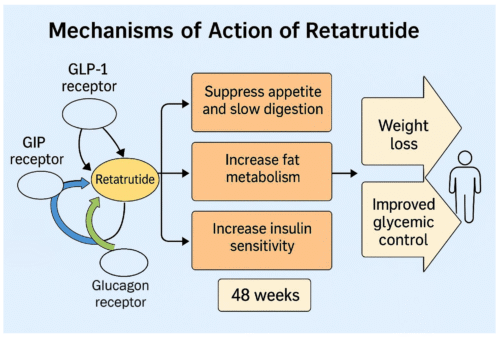
21 August 2025Retatrutide is an investigational weekly injection designed to help with weight management by acting on three hormone receptors at once: GLP-1, GIP and glucagon by a company called Eli Lilly. You can think of it as sending the body a coordinated set of signals that reduce hunger, improve how we handle food, and gently nudge us to burn more energy. Many people searching online ask how does retatrutide work for weight loss, how fast does retatrutide work, or even how quickly does retatrutide peptide work. Below is a plain-English explainer that addresses all these common questions.
Quick take: retatrutide combines appetite control (GLP-1), metabolic support and potential anti-nausea synergy (GIP), and a mild increase in energy expenditure (glucagon). In mid-stage trials it produced large average weight reductions, but it is not yet approved in the UK at the time of writing and remains under study.
It doesn’t have to be hard
It can be easy
First, a quick primer on the three hormones
To really answer the question “how does Retatrutide work”, it helps to look at the three different hormones it acts on. Each one plays a unique role in appetite, metabolism, and blood sugar regulation. By combining them, Retatrutide aims to create a broader, more powerful effect than older medicines that focus on just one or two pathways.
GLP-1 (glucagon-like peptide-1)
- Acts in the brain to curb appetite and cravings, so most people feel full sooner and snack less. This central effect is one of the main reasons GLP-1 drugs are so effective for weight control.
- Slows stomach emptying slightly, which means meals stay in the stomach for longer and provide a lasting sense of satiety. This helps reduce portion sizes without conscious restriction.
- Encourages the pancreas to release insulin only when glucose is high, helping to smooth out post-meal blood sugar spikes and lowering the risk of sharp highs and lows.
- GLP-1 signalling also appears to influence inflammation and cardiovascular risk factors, adding to its appeal for people with obesity and type 2 diabetes.
GIP (glucose-dependent insulinotropic polypeptide)
- Another “incretin” hormone released by the gut when we eat. In combination with GLP-1, GIP appears to amplify the brain’s recognition of fullness, further reducing food intake.
- It helps improve the way fat tissue handles nutrients. Instead of encouraging unhealthy fat storage, GIP may steer calories into safer metabolic pathways, potentially reducing harmful visceral fat.
- Some studies suggest that GIP activity can reduce nausea sometimes associated with GLP-1 therapy, which could make Retatrutide easier to tolerate than single-pathway drugs.
- In animal models, GIP has been linked to improved energy expenditure, hinting that it may play a role in burning calories more efficiently.
Glucagon
- Often thought of purely as a blood sugar–raising hormone, glucagon also has beneficial effects when balanced carefully with GLP-1 and GIP.
- It promotes the breakdown of stored fat and increases energy use, which can accelerate weight reduction when combined with appetite suppression.
- When paired with the insulin-boosting effects of GLP-1 and GIP, glucagon’s natural tendency to raise glucose can be balanced out—delivering fat-burning benefits without dangerous blood sugar spikes.
- This third hormone is what sets Retatrutide apart from earlier medicines, completing the “triple agonist” approach that explains how does Retatrutide work in a more comprehensive way than its predecessors.
Together, these three hormones form a coordinated network. GLP-1 helps people feel full and lowers glucose, GIP supports fat and nutrient handling, and glucagon pushes the body to burn energy. Retatrutide’s innovation is combining all three in one medicine.
Glucagon
- Best known as insulin’s counter-signal, but at the carefully tuned doses used in research it can increase calorie burning by raising energy expenditure and encouraging the body to use stored fat.
- Also communicates with the brain and liver to influence appetite and lipid handling.
Why combine them? On their own, GLP-1 medicines mainly reduce intake (you eat less). Adding GIP appears to reinforce appetite control and metabolic flexibility. Carefully titrated glucagon receptor activation may increase output (you burn a bit more). Retatrutide’s goal is to blend these effects into a single, steady weekly dose. This helps answer the common query, how does retatrutide work in the body.
How retatrutide works in practice
- Tames the drive to eat. Signals to appetite centres reduce hunger and cravings, which makes smaller portions feel comfortable. Many people naturally choose less-energy-dense foods when these circuits are calmer.
- Improves post-meal handling. With food on board, insulin release is more efficient and glucagon is kept in check. This steadies glucose and reduces the “spike-crash-snack” cycle that can undermine weight-loss efforts.
- Shifts the energy balance. Low-level glucagon receptor engagement nudges the body to oxidise fat and may raise resting energy expenditure modestly. That means you’re not only taking in fewer calories, you may also be using a fraction more.
- Weekly, long-acting dosing. Retatrutide is engineered with a long half-life, so you inject once a week with dose increases over time to improve tolerability. People often ask how quickly does retatrutide work—in studies, weight changes began in the first weeks and accumulated steadily over months.
What do clinical studies show so far?
Early- to mid-stage trials in people with obesity (with and without type 2 diabetes) have reported substantial average weight reductions over 48 weeks at higher doses, alongside improvements in measures like waist circumference, blood lipids and liver fat in those with metabolic fatty liver disease. These results suggest the triple-agonist approach can outperform single-hormone GLP-1 drugs, and even dual GLP-1/GIP agents in some analyses. Larger phase 3 trials are under way to confirm effectiveness and long-term safety.
Plain-English translation: in studies to date, many participants lost a significant proportion of their starting weight over about a year, and their metabolic markers generally moved in a healthier direction. This directly supports searches such as how does retatrutide work for weight loss or how does retatrutide work with weight lifting—the medicine reduces fat mass, while lifestyle measures like resistance training protect muscle.
Side effects and safety
Like other incretin-based medicines, the most common side effects are gastrointestinal: nausea, diarrhoea, constipation and occasional vomiting—usually milder with gradual dose increases and care around trigger foods (rich, spicy or very large meals). Small, temporary increases in resting heart rate have been observed in some participants during titration.
Important cautions shared across this drug class:
- Thyroid C-cell tumours in rodents: long-acting incretin drugs carry a rodent finding that leads to caution in people with a personal/family history of medullary thyroid carcinoma (MTC) or MEN2. Human relevance remains uncertain, but prescribers screen for risk.
- Gallbladder and pancreas: as with other weight-loss injections, report upper abdominal pain, persistent vomiting or jaundice promptly.
- Pregnancy and breastfeeding: not recommended; effective contraception is advised during use.
As with any new medicine, phase 3 trials and ongoing pharmacovigilance will define the real-world safety picture.
UK availability (summer 2025)
Retatrutide is not yet licensed in the UK for weight management. The NHS currently commissions other options (for example, semaglutide and Tirzepatide for eligible groups) under NICE guidance. Several phase 3 studies of retatrutide are in progress globally, including UK sites. If and when approvals happen, NHS access would follow a separate NICE appraisal.
Who might be considered in future, if approved?
If retatrutide is authorised, we’d expect criteria similar to current NHS policies for weight-management injections: a BMI threshold (with lower thresholds for some ethnic groups), presence of weight-related conditions (e.g., hypertension, OSA, prediabetes), and use alongside structured lifestyle support. Private prescriptions would follow MHRA licensing and professional guidelines.
Practical tips from early experience (if you’re ever prescribed a similar medicine)
- Titrate slowly and mind mealtimes. Small, regular meals; avoid very rich or greasy foods during dose increases.
- Protein and fibre matter. Prioritise lean protein and high-fibre foods to preserve muscle while losing fat.
- Hydration and electrolytes. Sip water through the day; consider a pinch of salt in food if light-headed.
- Keep moving. Gentle daily activity supports energy expenditure and helps with constipation.
- Track how you feel. If nausea, abdominal pain or sustained fast heart rate occur, contact your prescriber.
Key takeaways
- Three receptors, one injection: Retatrutide targets GLP-1 (less hunger), GIP (metabolic and satiety synergy) and glucagon (slightly more energy burn).
- Promising efficacy so far: Mid-stage trials show large average weight reductions and metabolic improvements, but phase 3 data are still pending.
- Familiar side-effect profile: Mostly gastrointestinal and dose-related; careful titration helps.
- Not yet available on the NHS: UK access awaits successful phase 3 results, regulatory approval and a positive NICE appraisal.
Frequently Asked Questions (FAQ)
Is Retatrutide available in the UK?
No. Retatrutide is still in clinical trials and is not available for prescription in the UK.
How is Retatrutide different from Mounjaro?
Mounjaro is a dual agonist (GLP-1 and GIP), while Retatrutide is a triple agonist (GLP-1, GIP, glucagon). This extra pathway may deliver greater weight loss.
What weight loss results have been seen so far?
In phase 2 trials, participants lost an average of ~24% of their body weight at 48 weeks on higher doses of Retatrutide.
When will Retatrutide be available?
Phase 3 results are expected in 2026. If successful, regulatory approval may follow afterwards.
This article is for general information only and is not medical advice. Always speak to a qualified clinician about your own circumstances.
